What is Stevia?


- Plant-based 100% natural sweetener extracted from leaves of stevia rebaudiana
- Zero calorie sugar substitute
- Potency of 200-400x the sweetness of sugar
- Approved by regulatory bodies and food safety experts worldwide and in all major markets including US, Europe, Canada, Australia/New Zealand, China, Japan, Korea, and many more
- Enjoyed by 5 billion people around the world in their food and beverages


The History of Stevia
Grown for centuries in Paraguay, the stevia rebaudiana plant has been used by many generations of indigenous South Americans as a natural sweetener for teas and medicines. Its taste was so powerful that some would even enjoy chewing on the plant’s leaves as nature’s sweet treat. It wasn’t until the 19th and 20th centuries though that scientists would discover and study these leaves, where they isolated and identified the sweet tasting chemical components as steviol glycosides. These compounds are the secret to stevia’s sweet magic, boasting a potency of 200-400x the sweetness of sugar. The stevia plant contains an abundant variety of these glycosides, each with their own sweetness and taste profile.
Today, stevia enhances the taste of food and beverage products enjoyed by 5 billion people around the globe. And centuries later, laboratories everywhere are still perfecting the science of extracting, refining and purifying steviol glycosides. SoPureTM’s research and development efforts are focused on unlocking the full potential of stevia by studying and analyzing the best applications for all its potential extracts. It is these efforts that have yielded SoPureTM the most complete and advanced portfolio of best tasting steviol glycosides available in the market.


Zero Calories. Limitless Potential.
Not only does stevia have a far higher sweetness potency than sugar, it also has none of sugar’s calories. The potential health benefits of lower caloric intake and reduced glycemic impact on blood sugar make stevia-based sweeteners an ideal sugar substitute for people with diabetes, children, and many others seeking healthier diets and lifestyles.
Stevia’s natural sweetness and potential health benefits are just some of the reasons its commercial use by food and beverage manufacturers has exploded — worldwide sales of high purity stevia are projected to reach close to $1 billion by 2027. Listed below are the many commercial characteristics of steviol glycosides that reveal the sweet and limitless potential of SoPureTM stevia.
- 100% all natural, non-artificial sweetener
- Pure and highly potent sweet taste
- Zero calories and zero glycemic index for healthier ingredients and healthier products
- Non-cariogenic and dental-friendly
- Versatility as a total or partial replacement for caloric sugars
- Flavor enhancer in use with other sweetener ingredients
- Heat stable up to about 390 F and can be used in cooking and baking as well as other high temperature processing and packaging conditions
- Extremely stable to low pH food, beverage processes and finished products systems
- Excellent solubility in aqueous systems


Regulatory Approval and Safety
Commercial use of stevia as a food and beverage sweetener first started in the 1970s in Japan. It wasn’t until more recent years that the adoption of stevia has surged in popularity around the world, after hundreds of long-term scientific studies confirmed that steviol glycosides are safe for human consumption. These safety conclusions paved the way for the Food and Agriculture Organization and the World Health Organization’s Joint Expert Committee on Food Additives (JECFA), a global panel of food ingredient safety experts, to approve the use of stevia in 2008 and 2009. In the US, the Food & Drug Administration (FDA) granted Generally Recognized As Safe (GRAS) status to high purity stevia extract in 2008. The same year, Food Standards Australia New Zealand (FSANZ) approved stevia as a food additive. The European Food Safety Authority (EFSA) also followed suit when they authorized the use of stevia in 2011.


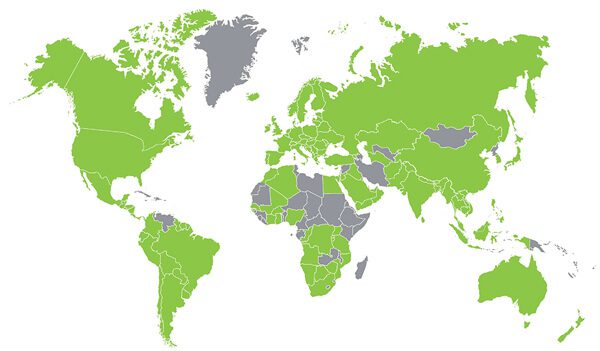
Where in the World is Stevia Approved?
Below is a global view of the countries in green where stevia has received regulatory approval.

SOURCE:
Global Stevia Institute
